Sunlight and sugarcane waste power hydrogen production at rate four times higher than commercialization benchmark
A technology for hydrogen (H2) production has been developed by a team of researchers led by Professors Seungho Cho and Kwanyong Seo from the School of Energy and Chemical Engineering at UNIST, in collaboration with Professor Ji-Wook Jang’s team from the Department of Materials Science and Engineering at UNIST.
Their research is published in the journal Nature Communications.
This innovative method utilizes biomass derived from sugarcane waste and silicon photoelectrodes to generate H2 exclusively using sunlight, achieving a production rate four times higher than the commercialization benchmark set by the U.S. Department of Energy (DOE).
H2 is recognized as a next-generation fuel since it emits no greenhouse gases when burned and stores energy at a density 2.7 times greater than gasoline. Despite this, the majority of H2 produced today is derived from natural gas, a process that generates substantial carbon dioxide emissions.
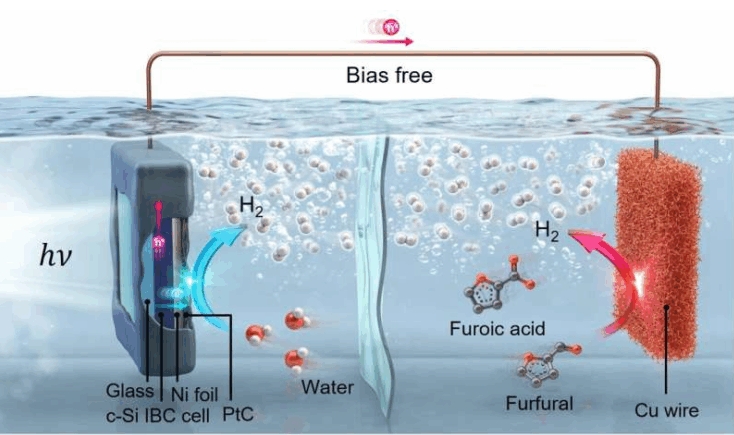
The research team has developed a photoelectrochemical (PEC) H2 production system that facilitates H2 production without carbon dioxide (CO2) emissions by utilizing furfural extracted from sugarcane waste.
In this system, furfural is oxidized at the copper electrode to produce H2, with the residual material converting into furoic acid, a high-value product.
H2 is produced at both electrodes in this system. At the opposing silicon photoelectrode, water is also split to yield H2. This dual production mechanism theoretically doubles the production rate compared to conventional PEC systems, with the actual performance reaching 1.4 mmol/cm2·h, nearly four times the U.S. Department of Energy’s target of 0.36 mmol/cm2·h.
The H2 production process begins when the photoelectrode absorbs sunlight and generates electrons. Crystalline silicon photoelectrodes are advantageous for H2 production due to their capacity to generate a significant number of electrons. However, the low voltage generated (0.6 V) makes it challenging to initiate H2 production reactions without external power.
The research team addressed this issue by introducing the oxidation reaction of furfural on the opposing electrode to balance the system’s voltage.
This innovative method utilizes biomass derived from sugarcane waste and silicon photoelectrodes to generate H2 exclusively using sunlight, achieving a production rate four times higher than the commercialization benchmark set by the U.S. Department of Energy (DOE).
H2 is recognized as a next-generation fuel since it emits no greenhouse gases when burned and stores energy at a density 2.7 times greater than gasoline. Despite this, the majority of H2 produced today is derived from natural gas, a process that generates substantial carbon dioxide emissions.
The research team has developed a photoelectrochemical (PEC) H2 production system that facilitates H2 production without carbon dioxide (CO2) emissions by utilizing furfural extracted from sugarcane waste.
In this system, furfural is oxidized at the copper electrode to produce H2, with the residual material converting into furoic acid, a high-value product.
H2 is produced at both electrodes in this system. At the opposing silicon photoelectrode, water is also split to yield H2. This dual production mechanism theoretically doubles the production rate compared to conventional PEC systems, with the actual performance reaching 1.4 mmol/cm2·h, nearly four times the U.S. Department of Energy’s target of 0.36 mmol/cm2·h.
The H2 production process begins when the photoelectrode absorbs sunlight and generates electrons. Crystalline silicon photoelectrodes are advantageous for H2 production due to their capacity to generate a significant number of electrons. However, the low voltage generated (0.6 V) makes it challenging to initiate H2 production reactions without external power.
The research team addressed this issue by introducing the oxidation reaction of furfural on the opposing electrode to balance the system’s voltage.
Additionally, this system employs an interdigitated back contact (IBC) structure to minimize voltage losses within the photoelectrode and wraps the electrode in nickel foil and glass layers to protect it from the electrolyte, ensuring long-term stability.
The submerged structure of the silicon photoelectrode provides a self-cooling effect, demonstrating superior efficiency and stability compared to external coupling structures, where the battery generating electricity through water decomposition and the electrolyzer producing H2 are separate entities.
Professor Jang stated,
This technology achieves an H2 production rate from solar energy that is four times higher than the commercialization standard set by the U.S.
“Department of Energy, playing a crucial role in enhancing the economic viability of solar H2 and ensuring competitive pricing against fossil fuel-based H2.”
Source: Hydrogencentral